氮
氮,原子序数7,原子量为14.006747。无色无臭气体.元素名来源于希腊文,原意是“硝石”。1772年由瑞典药剂师舍勒和英国化学家卢瑟福同时发现,后由法国科学家拉瓦锡确定是一种元素。氮在地壳中的含量为0.0046%,自然界绝大部分的氮是以单质分子氮气的形式存在于大气中,氮气占空气体积的78%。氮的最重要的矿物是硝酸盐。
理化特性
外观与性状: 无色无臭气体。
熔点(℃): -209.8
沸点(℃): -195.6
相对密度(水=1): 0.81(-196℃)
相对蒸气密度(空气=1): 0.97
饱和蒸气压(kPa): 1026.42(-173℃)
临界温度(℃): -147
临界压力(MPa): 3.40
原子体积:(立方厘米/摩尔)17.3
元素在太阳中的含量:(ppm) 1000
元素在海水中的含量:(ppm)
太平洋表面 0.00008
氮在空气中的密度是1.2572kg/m^3,在氨中的密度是1.2505kg/m^3
溶解性: 微溶于水、乙醇。
N原子的价电子层结构为2s2p3,即有3个成单电子和一对孤电子对,以此为基础,在形成化合物时,可生成如下三种键型:
氮有两种天然同位素:氮14和氮15,其中氮14的丰度为99.625%
|
最稳定的同位素 |
|
同位素 |
丰度 |
半衰期 |
衰变模式 |
衰变能量
MeV |
衰变产物 |
|
13N |
人造 |
9.965分钟 |
电子捕获 |
2.220 |
13C |
|
14N |
99.634 % |
稳定 |
|
15N |
0.366 % |
稳定 |
|
|
|
原子体积:(立方厘米/摩尔)17.3
元素在太阳中的含量:(ppm) 1000
元素在海水中的含量:(ppm)
太平洋表面 0.00008
氮在空气中的密度是1.2572kg/m^3,在氨中的密度是1.2505kg/m^3
元素名称:氮
元素符号:N
晶体结构:晶胞为六方晶胞。
氧化态:N-3, N-2, N-1, N+1, N+2, N+3, N+4, N+5
地壳中含量:(ppm)25
化学键能: (kJ /mol)
N-H 390
N-N 160
N=N 415
N≡N(氮气) 948
N-Cl 193
N-C 286
N=C 615
N≡C 887
晶胞参数:
a = 386.1 pm
b = 386.1 pm
c = 626.5 pm
α = 90°
β = 90°
γ = 120°
声音在其中的传播速率:(m/S)
353
热导率: W/(m·K)25.83
电离能 (kJ/ mol)
M - M+ 1402.3
M+ - M2+ 2856.1
M2+ - M3+ 4578.0
M3+ - M4+ 7474.9
M4+ - M5+ 9440.0
M5+ - M6+ 53265.6
M6+ - M7+ 64358.7
元素类型:非金属元素
元素原子量:14.01
质子数:7
中子数:7
原子序数:7
所属周期:2
所属族数:VA
电子层分布:L2-K5
氮气为无色、无味的气体,熔点-209.86°C,沸点-195.8°C,气体密度1.25046克/升,临界温度-146.95°C,临界压力33.54大气压。
氮通常的单质形态是氮气。它是无色无味无臭,十分不易有化学反应的原子的气体。而且它令火焰立刻熄灭。
制备
工业上常用低温分馏空气的办法把氮气和氧气分开。工业氮气都用黑色钢瓶装。
变压吸附:
原料空气经空气压缩机增压后,经过初级过滤器处理进入冷干机进行干燥处理,然后通过高效除油过滤器进入空气储罐,干燥、无油的洁净压缩空气先经过活性炭吸附塔,然后进入两只交换工作的吸附塔中,吸附塔中装有优质、高效的碳分子筛,由于氧和氮分子分子大小不一,在提高压力下分子筛优先吸附氧,一段时间后,分子筛对氧的吸附达到平衡,根据碳分子筛在不同压力下对吸附气体的吸附量不同的特性,降低压力使分子筛消除对氧的吸附,同时另一只吸附塔升压开始工作,通过两只吸附塔的交替工作获得连续的氮气
除低温分馏外,在工业上亦有使用分子筛碳(MSC)常压再生法(Pressure Swing Adsorption, PSA),来分离低纯度之氮气。
用途
廉价的惰性保护气,用于金属炼制及高温合成时的简单保护性氛围(其性能不及氦气及氩气);高温下用于合成氮化物(如氮化硅陶瓷、氮化硼等)。此外亦其化合物亦有用于农业,如氮肥。液态氮有时用于冷却。
氧化物
氮可以形成多种氧化物。
在氧化物中,氮的氧化数可以从+1到+5。
其中以NO和NO2较为重要。
氮的氧化物的性质如下表:
|
名称 |
化学式 |
状态 |
颜色 |
化学性质 |
熔点(℃) |
沸点(℃) |
|
一氧化二氮 |
N2O |
气态 |
无色 |
稳定,注:即是笑气 |
-90.8 |
-88.5 |
|
一氧化氮 |
NO |
气态 |
无色(固态、液态时为蓝色) |
反应能力适中 |
-163.6 |
-151.8 |
|
三氧化二氮 |
N2O3 |
液态 |
蓝色 |
室温下分解为NO和NO2 |
-102 |
-3.5(分解) |
|
二氧化氮 |
NO2 |
气态 |
红棕色 |
强氧化性 |
-11.2 |
21.2 |
|
四氧化二氮 |
N2O4 |
气态 |
无色 |
强烈地分解为NO2 |
-92 |
21.3 |
|
五氧化二氮 |
N2O5 |
固态 |
无色 |
不稳定 |
30 |
47(分解) |
由氧化剂(亚硝酸盐、重铬酸盐、氯气、溴、水、热CuO等)氧化氨及铵盐可得到氮气。实验室常用加热饱和亚硝酸钠和氯化铵溶液反应制取:
NH4Cl+NaNO2—→N2+2H2O+NaCl
用氨通过溴水也可制少量氮气:
8NH3+3Br2—→4N2+6NH4Br
由叠氮化钠(Sodium azida)NaN3热分解可得光谱纯N2:
2NaN3(s)—→2Na(l)+3N2(g)
3.1.2 氮的化学反应性
氮分子是由两个N原子以三个共价键相结合(∶N≡N∶),其电子结构为:

分子中存在一个σ键,两个π键,即叁重键(图3-1)
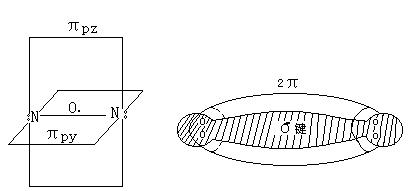
3-1 N2分子结构示意图
由表3-2所示数据,可知N≡N的键长短,键能很高946kJ·mol-1,要打破这样的三键很不容易,尤其是第三个键需能更高(531kJ·mol-1)。因此N2比任何其它双原子分子都稳定,在室温不与空气、水、酸反应,甚至在3273K时仅有1%离解。因此氮是化学惰性物质。氮的惰性广泛用于电子、钢铁、玻璃工业上作惰性复盖介质,还用于灯泡和可膨胀橡胶的填充物,工业上用于保护油类,保护粮食,在精密实验中用作保护气体。
表3-2氮的共价键键能与键长

在高温时氮的活泼性增强,与某些金属(Li、Mg、Ga、Al、B等)反应生成氮化物:
N2(g)+3Mg(s)—→Mg3N2(s)
氮与O2在高温(~2273K)或放电条件下直接化合成NO:
N2+O2→2NO,ΔrG  =173kJ·mol-1 =173kJ·mol-1
这是固定氮的一种方法。近年来有人用实验方法证实在闪电时空气中的氮和氧结合生成NO进而转化为硝酸随雨水降至地面为植物利用,是土壤氮的重要来源。
氮的主要用途是制氨,以及由此可得肥料、硝酸、炸药等重要化工原料。
3.1.3 氮的固定
氮是所有生命体系化学过程中的一个重要元素,也是粮食作物的决定因素。自然界中氮是取之不尽,用之不竭的。空气中含80%的氮,但以单质状态的氮却很难变成有用的化合物。因此把空气中的氮转化为可利用的含氮化合物即固氮(Nitrogen fixation)是人们十分关心的课题。
自然界的某些微生物和藻类,通过体内的一种具有特殊催化能力的蛋白质——固氮菌(酶)能将植物不能利用的空气中的氮素转化成可利用的氨态氮,如豆科植物大豆、花生的根瘤菌等。这种生物固氮作用对提高土壤肥力,保持自然界中氮素循环、节约资金和保护环境有极重要的意义。
长期以来,人们探索用化学方法把空气中的氮转化为氮的化合物,即人工固氮。人工固氮一般有三种:空气燃烧法(电弧法);氧、氮直接合成NO;氰氨基盐法,氮与CaC2加热至1073K形成氰氨化钙(CaCN2或CaNCN):
N2+CaC2-→CaCN2+C
氮与氢在高温高压催化剂条件下直接合成氨:
N2+3H3→2NH3 △rH  =-46kJ·mol-1 =-46kJ·mol-1
前两法消耗能量较大,目前广泛使用的氮氢合成氨法,但此法需具备高温高压和催化剂的合成条件。
人工固氮既消耗能量,而且产量也有限,据估计地球上每年生物固氮量约为2亿吨,相当于世界氮肥产量的4—5倍。可见生物固氮的能力极其强大。人们长期以来一直渴望着能用化学方法模拟固氮菌,实现在常温常压下固定空气中的氮制成氨。从60年代起开始了化学模拟生物固氮的研究,经研究证明固氮酶(铁、铁—钼蛋白质)中含有过渡金属与氮分子形成金属—氮分子配合物。这种配合物的形成使N2分子活化,易于被还原产生氨。从1965年第一个合成钌的氮分子配合物[Ru(NH3)5N2]X2(X=Cl-、Br-、I-、

Fe、Co、Ni、Ru、Rh、Ir、Pt等)—氮分子配合物。这证明了氮分子与CO相似能利用其孤对电子与过渡金属键合,形成过渡金属—氮分子配合物,这些配合物中的N2很易被还原为氨,如在可见光激发含有催化剂的Ru(Ⅱ)—N2配合物体系的水溶液,能使N2转化为氨。
最近又发现在常温下,用还原剂如VSO4或K2[MoO(CN)4(H2O)]和四氢硼酸盐在特定条件下能将N2还原至氨。这说明在常温下氮分子的惰性不是绝对的,在适宜的条件下是具有化学活性的。这些研究目前虽尚处在研究阶段,但为在常温常压下合成氨开辟了广泛的应用前景
nitrogen
N, atomic number 7, atomic weight is 14.006747. Odorless and colorless gas. Element name comes from the Greek and means "saltpeter." 1772 also found by the Swedish chemist Scheler and British chemist Rutherford, determined by the French scientist Lavoisier is an element. Nitrogen content in the crust is 0.0046%, most of the nitrogen in nature in the form of elemental nitrogen molecules in the atmosphere, nitrogen accounted for 78% of air volume. The most important mineral nitrogen are nitrates.
Physical and chemical properties
Appearance: colorless, odorless gas.
Melting point (℃): -209.8
Boiling point (℃): -195.6
Relative density (water = 1): 0.81 (-196 ℃)
Vapour Density (Air = 1): 0.97
Saturated vapor pressure (kPa): 1026.42 (-173 ℃)
Critical Temperature (℃): -147
Critical pressure (MPa): 3.40
:( Atomic volume cc / mol) 17.3
The content of elements in the sun: (ppm) 1000
Content elements in seawater: (ppm)
Pacific surface 0.00008
Nitrogen in the air density is 1.2572kg / m ^ 3, the density of ammonia is 1.2505kg / m ^ 3
Solubility: slightly soluble in water, ethanol.
Valence shell structure N atom 2s2p3, that is three to a single electron and one lone pair of electrons as a basis, in forming compounds, can generate the following three key types:
There are two natural isotopes of nitrogen: nitrogen 14 and nitrogen 15, where 14 is the abundance of nitrogen 99.625%
The most stable isotopes
Isotopic abundance of energy decay half-life decay mode
MeV decay products
13N artificial electron capture 9.965 minutes 2.220 13C
14N 99.634% stable
15N 0.366% stable
:( Atomic volume cc / mol) 17.3
The content of elements in the sun: (ppm) 1000
Content elements in seawater: (ppm)
Pacific surface 0.00008
Nitrogen in the air density is 1.2572kg / m ^ 3, the density of ammonia is 1.2505kg / m ^ 3
Element name: Nitrogen
Element symbol: N
Crystal structure: unit cell is hexagonal unit cell.
Oxidation state: N-3, N-2, N-1, N + 1, N + 2, N + 3, N + 4, N + 5
Content of the crust: (ppm) 25
Bond energy: (kJ / mol)
N-H 390
N-N 160
N = N 415
N≡N (nitrogen) 948
N-Cl 193
N-C 286
N = C 615
N≡C 887
Cell parameters:
a = 386.1 pm
b = 386.1 pm
c = 626.5 pm
α = 90 °
β = 90 °
γ = 120 °
Sound propagation speed in which: (m / S)
353
Thermal conductivity: W / (m · K) 25.83
Ionization energy (kJ / mol)
M - M + 1402.3
M + - M2 + 2856.1
M2 + - M3 + 4578.0
M3 + - M4 + 7474.9
M4 + - M5 + 9440.0
M5 + - M6 + 53265.6
M6 + - M7 + 64358.7
Element types: non-metallic elements
Elements of atomic weight: 14.01
Proton: 7
Neutron: 7
Atomic number: 7
Respective period: 2
Number of affiliated group: VA
Electron shell distribution: L2-K5
Nitrogen is a colorless, odorless gas, the melting point of -209.86 ° C, the boiling point of -195.8 ° C, the gas density 1.25046 g / l, the critical temperature of -146.95 ° C, critical pressure of 33.54 atmospheres.
Nitrogen is the usual form of elemental nitrogen. It is colorless, odorless, very difficult to have a chemical reaction of the gas atoms. And it makes the flame goes out immediately.
preparation
Commonly used in industry cryogenic distillation of air way to separate nitrogen and oxygen. Industrial nitrogen are fitted with black cylinders.
PSA:
After the feed air compressor pressurized air through the primary filter processing into the cold and dry machine for drying, and then into the air reservoir through efficient oil filter, dry, clean and oil-free compressed air through the activated carbon adsorption tower first, then work into the two exchange adsorption tower, absorption tower equipped with high quality and efficiency of the carbon molecular sieve oxygen and nitrogen molecules due to the molecular sizes, under elevated pressure sieve preferentially adsorbs oxygen, after a period of time, molecular sieve adsorption of oxygen balance, according to the different carbon molecular sieve under pressure in the absorption amount of the different characteristics of gas, reducing the pressure to eliminate molecular sieve adsorption of oxygen, while the other adsorber boost to work through two adsorbers working alternately obtained continuous nitrogen
In addition to low temperature fractionation, but also in the industrial use of carbon molecular sieve (MSC) regeneration pressure (Pressure Swing Adsorption, PSA), to separate the low purity of nitrogen.
use
Cheap inert gas protection, protective atmosphere for simple metal refining and high-temperature synthesis of (its performance is less than helium and argon); high temperature for the synthesis of nitride (such as silicon nitride ceramics, boron nitride, etc.) . In addition it also compound also used in agriculture, such as nitrogen. Sometimes liquid nitrogen for cooling.
Oxide
Nitrogen oxides can form a variety.
In the oxide, nitrogen oxide number may range from +1 to +5.
Among the more important of NO and NO2.
The nature of nitrogen oxides in the following table:
Name Chemical Formula status colors and chemical properties Melting point (℃) Boiling point (℃)
Nitrous oxide, N2O gas colorless stable Note: that is, nitrous oxide -90.8 -88.5
NO colorless gaseous nitric oxide (solid, liquid when blue) -163.6 -151.8 moderate response capability
Dinitrogen trioxide N2O3 blue liquid at room temperature decomposition of NO and NO2 -102 -3.5 (decomposition)
Nitrogen dioxide NO2 gaseous red-brown strong oxidizing -11.2 21.2
Dinitrogen tetroxide N2O4 strongly colorless gaseous decomposition NO2 -92 21.3
Dinitrogen pentoxide unstable colorless solid N2O5 3047 (decomposition)
Oxidant (nitrite, dichromate, chlorine, bromine, water, heat CuO, etc.) oxidation of ammonia and ammonium nitrogen available. Commonly used laboratory heating saturated solution of sodium nitrite and ammonium chloride from the reaction:
NH4Cl + NaNO2- → N2 + 2H2O + NaCl
Ammonia can also be prepared by using a small amount of nitrogen Bromine:
8NH3 + 3Br2- → 4N2 + 6NH4Br
By the sodium azide (Sodium azida) NaN3 thermal decomposition can be obtained spectroscopically pure N2:
2NaN3 (s) - → 2Na (l) + 3N2 (g)
3.1.2 chemically reactive nitrogen
Nitrogen molecule is composed of two to three N atoms covalently combined (:N≡N:), the electronic structure of:
A σ bond present in the molecule, two π bond, i.e. triplet key (3-1)
3-1 N2 molecular structure diagram
The data shown in Table 3-2, it was found N≡N key length, high bond energy 946kJ · mol-1, to break this triple bond is not easy, especially in the third key demand higher energy (531kJ · mol-1). Therefore N2 than any other diatomic molecules are stable, no air, water, acid reaction, even just at 3273K 1% dissociation at room temperature. Nitrogen is therefore chemically inert material. Inert nitrogen is widely used as the inert media coverage on electronics, steel, glass industry, but also for light bulbs and inflatable rubber filler, for the protection of industrial oils, food protection, as a protective gas in precision experiments .
Table 3-2 nitrogen covalent bond with bond lengths
Active at high temperatures nitrogen enhancement, and some metal (Li, Mg, Ga, Al, B, etc.) to form a nitride:
N2 (g) + 3Mg (s) - → Mg3N2 (s)
Nitrogen and O2 at high temperatures (~ 2273K) or direct discharge conditions of synthesis of NO:
N2 + O2 → 2NO, ΔrG = 173kJ · mol-1
This is a method of nitrogen fixation. In recent years, it was experimentally confirmed that when lightning atmospheric nitrogen and oxygen combine to generate NO is converted to nitrate with the rain and then lowered to the ground for the plant use, is an important source of nitrogen in the soil.
The main purpose of nitrogen is ammonia, and thus the available fertilizer, nitrate, explosives, and other important chemical raw materials.
3.1.3 fixed nitrogen
Nitrogen is a chemical process in all living systems is an important element in deciding factor is food crops. Nitrogen in nature is inexhaustible. Air contains 80% of nitrogen, the nitrogen is difficult elemental state into useful compounds. Therefore, the nitrogen in the air into nitrogen-containing compound can be used, namely nitrogen fixation (Nitrogen fixation) is very concerned about the people issue.
Some algae and micro-organisms in nature, the protein has a special catalytic capabilities by means of a body - nitrogen-fixing bacteria (enzyme) plant can not use nitrogen in the air is converted to ammonia nitrogen can be used, such as legumes soybean, peanut and other rhizobia. Such biological nitrogen fixation to improve soil fertility and maintain nitrogen cycling in nature, save money and protect the environment has a very important significance.
For a long time, people used chemicals to explore the atmospheric nitrogen into nitrogen compounds that artificial nitrogen fixation. Artificial nitrogen fixation are generally three types: combustion air (arc method); oxygen, nitrogen direct synthesis of NO; dicyanamide salt method, nitrogen and heated to 1073K CaC2 formed calcium cyanamide (CaCN2 or CaNCN):
N2 + CaC2- → CaCN2 + C
Nitrogen and hydrogen at elevated temperature and pressure conditions for direct ammonia catalyst:
N2 + 3H3 → 2NH3 △ rH = -46kJ · mol-1
The first two methods energy consumption is large, the current widespread use of nitrogen hydrogen ammonia method, but this method need to have high temperature and pressure synthesis conditions and the catalyst.
Artificial nitrogen fixation both energy consumption and production is limited, it is estimated that each year about 200 million tons of biological nitrogen fixation on the planet, equivalent to 4-5 times the world fertilizer production. Visible biological nitrogen fixation ability is extremely powerful. It has long been eager to use chemical methods simulate nitrogen-fixing bacteria, made of ammonia nitrogen to achieve under normal temperature and pressure fixed air. From the 1960s began to study chemistry simulation of biological nitrogen fixation, the study proved that nitrogenase enzyme (iron, iron - molybdenum protein) containing a transition metal and nitrogen molecules to form metal - nitrogen molecule complex. Such complexes are formed so that N2 molecule activation, easy to be produced by the reduction of ammonia. From 1965 the first synthetic nitrogen molecule ruthenium complex [Ru (NH3) 5N2] X2 (X = Cl-, Br-, I-,
Fe, Co, Ni, Ru, Rh, Ir, Pt, etc.) - N molecular complex. This demonstrates similar molecular nitrogen and CO can use its lone pair of electrons and bonded to the transition metal, a transition metal is formed - molecular nitrogen complex, these complexes are very easily reduced to N2 of ammonia, as a catalyst in the visible light excitation containing Ru aqueous solution (ⅱ) -N2 complex system, enabling N2 into ammonia.
Recently found that at room temperature, with a reducing agent such as VSO4 or K2 [MoO (CN) 4 (H2O)] and tetrahydro borate can N2 reduction to ammonia under certain conditions. This shows that at room temperature inert nitrogen molecules is not absolute, under appropriate conditions is a chemically active. These studies at present, although still at the research stage, but at normal temperature and pressure of ammonia opens up a wide range of applications |