|
|
|
| 设备 >> 氢、氢气、Hydrogen,Hydrogen, |
 |
| |
|
产品编号: |
1215131716 |
产品名称: |
氢、氢气、Hydrogen,Hydrogen, |
规 格: |
1 |
产品备注: |
氢,氢气,氢气纯化,制氢、Hydrogen, hydrogen, hydrogen purification, hydrogen production, |
产品类别: |
设备 |
| |
|
|
|
氢 Hydrogen,
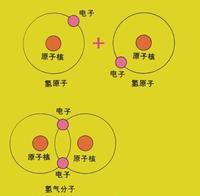
氢在通常条件下为无色、无味的气体;气体分子由双原子组成;熔点-259.14°C,沸点-252.8°C,临界温度33.19K,临界压力12.98大气压,气体密度0.0899克/升;水溶解度21.4厘米³/千克水(0°C),稍溶于有机溶剂。
在常温下,氢比较不活泼,但可用合适的催化剂使之活化。在高温下,氢是高度活泼的。除稀有气体元素外,几乎所有的元素都能与氢生成化合物。非金属元素的氢化物通常称为某化氢,如卤化氢、硫化氢等;金属元素的氢化物称为金属氢化物,如氢化锂、氢化钙等。
氢是重要的工业原料,又是未来的能源,也是最清洁的燃料.
元素在太阳中的含量:(ppm) 7500000
地壳中含量:(ppm)1500
在常温下,氢气比较不活泼,但可用催化剂活化。单个存在的氢原子则有极强的还原性。在高温下氢非常活泼。除稀有气体元素外,几乎所有的元素都能与氢生成化合物。
名称, 符号, 序号:氢、H、1
系列:非金属
原子体积:(立方厘米/摩尔)14.4
氧化态:Main H+1 Other H0, H-1
族, 周期, 元素分区:1族, 1, s
电离能 (kJ /mol)
M - M+ 1312
密度、硬度:0.0899 kg/m3(273K)、NA
热导率: W/(m·K)180.5
化学键能: (kJ /mol)
H-H 454
H-F 566
H-Cl 431
H-Br 366
H-I 299
晶胞参数:
a = 470 pm
b = 470 pm
c = 340 pm
α = 90°
β = 90°
γ = 120°
颜色和外表:无色
声音在其中的传播速率:(m/S)1310
Image:H,1.jpg
大气含量:0.0001 %
地壳含量:0.88 %
原子属性
原子量:1.00794 原子量单位
原子半径:(计算值) 25(53)pm
共价半径:37 pm
范德华半径:120 pm
价电子排布:1s1
电子在每能级的排布:1
氧化价(氧化物):1(两性的)
晶体结构:六角形
物质状态 气态
核内质子数:1
核外电子数:1
核电核数:1
质子质量:1.673E-27
质子相对质量:1.007
所属周期:1
所属族数:IA
摩尔质量:1g/mol
氧化物:H2O
最高价氧化物:H2O
外围电子排布:1s1
核外电子排布:1
颜色和状态:无色气体
原子半径:0.79
常见化合价:+1,-1
熔点:14.025 K (-259.125 °C)
沸点:20.268 K (-252.882 °C)
摩尔体积:22.4L/mol
汽化热:0.44936 kJ/mol
熔化热:0.05868 kJ/mol
蒸气压:209 帕(23K)
声速:1270 m/s(293.15K)
电负性:2.2(鲍林标度)
比热:14304 J/(kg·K)
电导率:无数据
热导率:0.1815 W/(m·K)
电离能:1312 kJ/mol
最稳定的同位素
同位素 丰度 半衰期 衰变模式 衰变能量
MeV 衰变产物
1H 99.985 % 稳定
2H 0.015 % 稳定
3H 10-15 % /
人造 12.32年 β衰变 0.019 3He
4H 人造 9.93696×10-23秒 中子释放 2.910 3H
5H 人造 8.01930×10-23秒 中子释放 4H
6H 人造 3.26500×10-22秒 三粒中子
释放 ? 3H
7H 人造 无数据 中子释放? ? 6H?
核磁公振特性
1H 2H 3H
核自旋 1/2 1 1/2
灵敏度 1 0.00965 1.21
在地球上和地球大气中只存在极稀少的游离状态氢。在地壳里,如果按重量计算,氢只占总重量的1%,而如果按原子百分数计算,则占17%。氢在自然界中分布很广,水便是氢的“仓库”——水中含11%的氢;泥土中约有1.5%的氢;石油、天然气、动植物体也含氢。在空气中,氢气倒不多,约占总体积的一千万分之五。在整个宇宙中,按原子百分数来说,氢却是最多的元素。据研究,在太阳的大气中,按原子百分数计算,氢占81.75%。在宇宙空间中,氢原子的数目比其他所有元素原子的总和约大100倍。
工业制法:电解水 2H2O=O2↑+2H2↑
实验室制法:锌与稀盐酸反映 Zn+2HCl=ZnCl2+H2↑
其他制法:Fe+H2SO4=FeSO4+H2↑
Mg+H2SO4=MgSO4+H2↑
2Al+3H2SO4=Al2(SO4)3+H2↑
Fe+2HCl=FeCl2+H2↑
Mg+2HCl=MgCl2+H2↑
Zn+2HCl=ZnCl2+H2↑
2Al+6HCl=2AlCl3+3H2↑
工业法有电解法、烃裂解法、烃蒸气转化法、炼厂气提取法。
纯化
随着半导体工业、精细化工和光电纤维工业的发展,产生了对高纯氢的需求。例如,半导体生产工艺需要使用99.999%以上的高纯氢。但是目前工业上各种制氢方法所得到的氢气纯度不高,为满足工业上对各种高纯氢的需求,必须对氢气进行进一步的纯化。氢气的纯化方法大致可分为两类(物理法和化学法),六种方法。
氢气的纯化方法:
|
方法 |
基本原理 |
适用原料气 |
制得的氢气纯度(%) |
适用规格 |
|
高压催化法 |
氢与氧发生催化反应而除去氧 |
含氧的氢气,主要为电解法制得的氢气 |
99.999 |
小 |
|
金属氢化物分离法 |
先使氢与金属形成金属氢化物后,加热或减压使其分解 |
氢含量较低的气体 |
>99.9999 |
中小 |
|
高压吸附法 |
吸附剂选择吸附杂质 |
任何含氢气体 |
99.999 |
大 |
|
低温分离法 |
低温下使气体冷凝 |
任何含氢气体 |
90~98 |
大 |
|
钯合金薄膜扩散法 |
钯合金薄膜对氢有选择渗透性,而其他气体不能透过 |
氢含量较低的气体 |
>99.9999 |
中小 |
|
聚合物薄膜扩散法 |
气体通过薄膜的扩散速率不同 |
炼油厂废气 |
92~98 |
小 |
用途
氢是重要工业原料,如生产合成氨和甲醇,也用来提炼石油,氢化有机物质作为收缩气体,用在氢氧焰熔接器和火箭燃料中。在高温下用氢将金属氧化物还原以制取金属较之其他方法,产品的性质更易控制,同时金属的纯度也高。广泛用于钨、钼、钴、铁等金属粉末和锗、硅的生产。
由于氢气很轻,人们利用它来制作氢气球。氢气与氧气化合时,放出大量的热,被利用来进行切割金属。
利用氢的同位素氘和氚的原子核聚变时产生的能量能生产杀伤和破坏性极强的氢弹,其威力比原子弹大得多。
现在,氢气还作为一种可替代性的未来的清洁能源,用于汽车等的燃料。为此,美国于2002年还提出了“国家氢动力计划”。但是由于技术还不成熟,还没有进行大批的工业化应用。2003年科学家发现,使用氢燃料会使大气层中的氢增加约4~8倍。认为可能会让同温层的上端更冷、云层更多,还会加剧臭氧洞的扩大。但是一些因素也可抵销这种影响,如使用氯氟甲烷的减少、土壤的吸收、以及燃料电池的新技术的开发等。
比金子还要贵的水
前面介绍的是普通的氢,它的原子量是1,它还有两个“能干”的大“哥哥”氘(音刀)和氚(音川)它们的原子量分别是2和3。人们有时候也把它们称为“重氢”和“超重氢”,它们与氧结合生成的水分别叫重水和超重水。
水在地球上的总重大约是140亿亿吨,其中重水还不到万分之二。为了得到一公斤重水就要消耗掉6万度电和一百吨水,这比砂里淘金花的代价要大得多,因而重水的价格要比金子贵。大自然中的重水非常少,而超重水就更加少了,在宽广无际的大海里,连十亿分之一也找不到,只有靠人工的方法去制造。一般是把金属锂放在原子反应堆中,在中子的轰击下,使锂转变为氚,然后与氧化合生成超重水。制造一公斤超重水要消耗近十吨的原子能量,而且生产很慢,一个工厂一年也不过制造几十公斤超重水,所以超重水的价格比重水还要贵上万倍,比金子要贵几十万倍。
表面看来,重水和一般的水没有什么两样。但脾气却大不一样,如果你用重水养金鱼,没多久鱼便死了,用重水浸过的种子不会发芽。重水的“个头”也比水大,一立方米重水比一立方米普通的水要重105.6公斤。普通的水在零度时结冰,在100℃时沸腾;而重水在3.8℃时就变成了冰,人们把它叫做“热冰”。
虽然重水和超重水生产起来要花费很大代价,但人们还是在不断地制造着他们。这是什么缘故呢?
原来它们对人类也有很多好处。先说起重水,它有放射性,利用它的这个特性,科学家可以研究某些生物或化学过程的进展情况。比如让病人喝一点含有极少量超重水的茶,半小时后,就可以从尿中检查出放射性,一直到14天以后,放射性才消失,这说明水分在人体中停留的时间是14天。如果要研究某种化学过程中水的来龙去脉,但又不许加入别的东西来破坏化学反应,这时就可以在普通水中加入一些超重水,超重水流到哪儿,哪儿就出现放射性。科学家很容易用探测器测量出它的藏身之处。
重水是原子能工业中的重要角色,它是原子反应堆最好的减速剂和载热剂,用了它之后,就可以大大降低原子燃料的成分。重水还是重要的国防原料,氢弹就是用它来制造的,重氢在极高温度下会产生原子核的聚合反应,发生强烈的爆炸,它的能量相当于几千万吨烈性炸药。一个普通的氢弹就能轻而易举地炸毁一座城市。如果把它爆炸时放出能量全部转换成电能,人类几十年也用不完!
安全
氢是在已知气体中最轻的气体。氢在常温常压下是无色无臭无味的可燃性气体。它除因缺氧而引起窒息外,还没有发现毒性。氢与空气、氧、卤素的亲和力强。氢气在空气和氧气中有很宽的可燃范围。氢气的燃点较高,但其点火能很小,所能很容易着火,在微小的静电火花下也容易着火。这是一个具有特殊意义的性质。当它接触明火或遇热时就可燃烧,而且发出几乎看不见的火焰。氢气又是一种高能燃料,当与空气或其它氧化剂结合着火时,以放热或爆炸的方式释放出大量的能量,其反应的猛烈程度取决于燃烧的条件。氢与卤素气体的混合物在日光下也能发生爆炸。氢与一氧化二氮的混合物的爆炸范围为5.2%~80%,与一氧化氮混合物的爆炸范围为13.5%~49%。氢气的这一易燃易爆性是极其危险可怕的特性。氢又是很容易扩散和浸透的气体,它非常容易泄漏,而且好停留在天花板等高处。
低温氢气与常温氢气密度不同,当它从液态氢开始蒸发时比空气重,沉积在地面上,等升温后才开始扩散。冷氢气遇到潮湿空气时能形成浓雾,并由此可看出它扩散的迹象。但在可见到的浓雾外围仍能形成爆炸性混合物。如果氢气云在最初闪速蒸发时着火,就会产生火球。
氢的还原性很强,在高温与金属氧化物、金属氯化物反应游离出金属,所以它一般没有腐蚀性。在白金等催化剂的作用下与有机化合物作用还原醛等不饱和烃。
C2H2+H2—→C2H4
C2H4+H2—→C2H6
CH3CHO+H2—→CH3CH2OH
CH3COOC2H5+2H2—→2CH3CH20H
CH3COCH3+H2—→CH3CH—CH3
| OH
氢又能浸入金属的晶格之间使晶格膨胀或变形,造成金属材料的脆化。钢材在高温下产生如下脱碳反应而受氢的浸蚀。
Fe3C+2H2—→CH4+3Fe
氢气能被过渡金属可逆性地吸附或吸收而生成不定量的金属氢化物。被吸收的氢气的量在Pd、Pt、Ni、Ti、Fe等中较多,对Pd来说,它能吸收其体积800倍的氢气。
氢微溶于水,在20℃时吸收系数a为0.0182。
5.毒性
氢气本身无毒,吸入后仍以原形排出。它是一种窒息剂,但是在实际工作中因氢气窒息而死亡的例子很少,然而,因氢气引起的火灾和爆炸事故却不断地发生。因此对氢气的易燃易爆性应引起足够地重视。
氢气一般充装在高压钢瓶或液化后装在低温容器中。所以除了高压氢气的泄漏起火或爆炸的危险之外,还有被液氢冷烧伤的危险。
Under normal conditions hydrogen is a colorless, odorless gas; gas molecules by a double atoms; melting point -259.14 ° C, the boiling point of -252.8 ° C, the critical temperature 33.19K, the critical pressure of 12.98 atmospheres, gas density 0.0899 g / l; water solubility 21.4 cm & sup3; / kg water (0 ° C), slightly soluble in organic solvents.
At room temperature, hydrogen relatively inactive, but can be used to make a suitable catalyst activation. At high temperatures, hydrogen is highly reactive. In addition to the rare gas element, almost all of the elements can form compounds with hydrogen. Hydrides is generally called a nonmetallic element hydrogen, such as hydrogen halide, hydrogen sulfide and the like; metal hydride element called metal hydrides, such as lithium hydride, calcium hydride.
Hydrogen is an important industrial raw material, but also the future of energy is the cleanest fuel.
The content of elements in the sun: (ppm) 7500000
Content of the crust: (ppm) 1500
At room temperature, hydrogen relatively inert, but can be the catalyst activation. The existence of a single hydrogen atom, there are strong reductive. Hydrogen at high temperatures is very lively. In addition to the rare gas element, almost all of the elements can form compounds with hydrogen.
Names, symbols, ID: hydrogen, H, 1
Series: Non-metallic
:( Atomic volume cc / mol) 14.4
Oxidation states: Main H + 1 Other H0, H-1
Family, period, zoning elements: Group 1, 1, s
Ionization energy (kJ / mol)
M - M + 1312
Density, Hardness: 0.0899 kg / m3 (273K), NA
Thermal conductivity: W / (m · K) 180.5
Bond energy: (kJ / mol)
H-H 454
H-F 566
H-Cl 431
H-Br 366
H-I 299
Cell parameters:
a = 470 pm
b = 470 pm
c = 340 pm
α = 90 °
β = 90 °
γ = 120 °
Color and Appearance: colorless
Sound propagation speed in which: (m / S) 1310
Image: H, 1.jpg
Atmospheric content: 0.0001%
Crustal content: 0.88%
Atomic Properties
Atomic weight: 1.00794 atomic mass units
Atomic radius :( calc) 25 (53) pm
Covalent radius: 37 pm
Van der Waals radius: 120 pm
Valence electron arrangement: 1s1
Electronic energy levels in each arrangement: 1
Price oxide (oxide): 1 (bisexual)
Crystal structure: Hexagonal
Physical Properties
Gaseous state of matter
Number of protons in the nucleus: 1
Extranuclear: 1
Nuclear audit: 1
Proton mass: 1.673E-27
Proton relative quality: 1.007
Respective period: 1
Number of affiliated group: IA
Molar mass: 1g / mol
Oxides: H2O
Highest price oxides: H2O
External electronic arrangement: 1s1
Extranuclear electron arrangement: 1
Colors and states: colorless gas
Atomic radius: 0.79
Common valence: +1, -1
Melting point: 14.025 K (-259.125 ° C)
Boiling point: 20.268 K (-252.882 ° C)
Molar volume: 22.4L / mol
Heat of vaporization: 0.44936 kJ / mol
Heat of fusion: 0.05868 kJ / mol
Vapor Pressure: 209 Pa (23K)
Sound velocity: 1270 m / s (293.15K)
Other properties
Electronegativity: 2.2 (Pauling scale)
Specific heat: 14304 J / (kg · K)
Conductivity: No data
Thermal conductivity: 0.1815 W / (m · K)
Ionization energy: 1312 kJ / mol
The most stable isotopes
Isotopic abundance of energy decay half-life decay mode
MeV decay products
1H 99.985% stable
2H 0.015% stable
3H 10-15% /
Artificial 12.32 on β decay 0.019 3He
4H artificial 9.93696 × 10-23 seconds neutron release 2.910 3H
5H artificial 8.01930 × 10-23 seconds neutron release 4H
6H artificial 3.26500 × 10-22 seconds three neutrons
Release? 3H
7H no artificial neutron data release?? 6H?
Nuclear magnetic resonance characteristic public
1H 2H 3H
Nuclear spin 1/2 1 1/2
Sensitivity 1 0.00965 1.21
distributed
The Earth and the atmosphere there is only a very few of the free state hydrogen. In the Earth's crust, if calculated according to weight, hydrogen is only 1% of the total weight, and if the calculated atomic percent, accounted for 17%. Hydrogen is widely distributed in nature, hydrogen is water "warehouse" - water containing 11% hydrogen; soil about 1.5% hydrogen; oil, natural gas, hydrogen also animals and plants. In the air, the hydrogen down much, of the total volume of 5/10000000. In the universe as a whole, in terms of atomic percentage, it is the most elemental hydrogen. According to the research, in the sun's atmosphere, the atomic percentage basis, accounting for 81.75% of hydrogen. In space, the number of hydrogen atoms and about 100 times larger than the total of all other elements of atoms.
preparation
Industrial production method: electrolysis of water 2H2O = O2 ↑ + 2H2 ↑
Laboratory Method: zinc and dilute hydrochloric reflect Zn + 2HCl = ZnCl2 + H2 ↑
Other Method: Fe + H2SO4 = FeSO4 + H2 ↑
Mg + H2SO4 = MgSO4 + H2 ↑
2Al + 3H2SO4 = Al2 (SO4) 3 + H2 ↑
Fe + 2HCl = FeCl2 + H2 ↑
Mg + 2HCl = MgCl2 + H2 ↑
Zn + 2HCl = ZnCl2 + H2 ↑
2Al + 6HCl = 2AlCl3 + 3H2 ↑
Industry Act electrolytic method, hydrocarbon cracking method, a method of hydrocarbon steam reforming, refinery gas extraction.
purification
With the development of the semiconductor industry, chemical industry and electric and optical fiber, resulting in a demand for high-purity hydrogen. For example, the semiconductor manufacturing process requires the use of more than 99.999% purity. But now all kinds of industrial hydrogen purity hydrogen production methods obtained is not high, in order to meet the demand for a variety of high purity hydrogen industry, the need for further purification of hydrogen gas. Hydrogen purification method can be broadly divided into two categories (physical and chemical methods), six methods.
Hydrogen purification method:
The method applies the basic principles of a feed gas obtained hydrogen purity (%) Applicable Standard
High-pressure catalytic catalytic reaction of hydrogen and oxygen and hydrogen to remove the oxygen-containing oxygen, mainly for electrolytic method of small hydrogen 99.999
Metal hydride hydrogen separation prior to the metal to form a metal hydride, followed by heating under reduced pressure or decomposed low hydrogen content gas> 99.9999 Small
High-pressure adsorption adsorbent selective adsorption of any impurity containing gas 99.999 big
Low temperature cryogenic separation of gas condensate any hydrogen-containing gas 90 to 98 large
Palladium alloy film diffusion method palladium alloy film selectively permeable to hydrogen, and other gases impermeable lower hydrogen content gas> 99.9999 Small
Polymer film diffusion method of gas diffusion rate through the film different refinery off-gas from 92 to 98 small
use
Hydrogen is an important industrial raw materials, such as the production of synthetic ammonia and methanol is also used to extract oil, hydrogenated organic substance as a contraction of the gas used in oxyhydrogen flame welding and rocket fuel. At elevated temperature with hydrogen reduction of metal oxides in the preparation of metal compared to other methods, the nature of the product easier to control, while the purity of the metal is also high. Widely used in tungsten, molybdenum, cobalt, germanium, and metal powder such as iron, silicon production.
Because hydrogen is very light, people use it to produce hydrogen balloon. When hydrogen and oxygen compounds and emit a lot of heat, being used to cut metal.
Use energy generated during nuclear fusion of hydrogen isotopes deuterium and tritium atoms can produce destruction and devastating hydrogen bomb, its much more powerful than the atomic bomb.
Now, hydrogen is also used as future clean energy one kind of substitutability for automobile fuel. To this end, the United States in 2002, also proposed a "national hydrogen program." But because technology is not mature, not yet conducted a large number of industrial applications. 2003 scientists discovered that the use of hydrogen fuel hydrogen atmosphere will increase by about 4 to 8 times. Think might make the same upper stratosphere colder, more clouds, but also aggravate the ozone hole. But some factors can offset this effect, reduce the use of chlorofluorocarbons such as methane, absorb the soil, and the development of new technologies such as fuel cells.
More expensive than gold water
Described above are ordinary hydrogen, its atomic weight is 1, it has two "do" big "brother" deuterium (sound knives) and tritium (Tone River) their atomic weight is 2 and 3 respectively. People sometimes refer to them as "heavy hydrogen" and "tritium", which combine to generate water and heavy water not call overweight water and oxygen.
The total water on Earth weighs about 14 billion tons, of which less than ten thousandths of heavy water. In order to obtain one kilogram of heavy water will consume 60,000 kWh of electricity and 101 tons of water, which is more than the cost of sand in the gold flower is much greater, and therefore the price of heavy water than Jin Zigui. Nature of the heavy water is very small, and even more overweight and less water in the wide expanse of the sea, one can not find even a billionth only by artificial means to manufacture. Generally the lithium metal in atomic reactors, under neutron bombardment, the lithium into tritium, then combines with oxygen to generate excess water. Manufacture one kilogram overweight tons of water to consume nearly atomic energy, and the production is very slow, but also a factory manufacturing year tens of kilograms overweight water, so the price of water overweight proportion of water is more expensive on the times, more expensive than gold dozens of times.
On the surface, heavy water and general water is no different. But temper quite different, if you use heavy water goldfish, not long after the fish will die, with heavy water soaked seeds will not germinate. Heavy water "head" than the big water, one cubic meter of heavy water than one cubic meter of water for a normal weight 105.6 kg. Ordinary water freezes at zero degrees, boiling at 100 ℃; and heavy water at 3.8 ℃ becomes ice, people call it "hot ice."
Although the production of heavy water and excess water takes up a great price, but people still continue to manufacture them. What happen?
They turned out to humans, there are many benefits. Speaking first heavy water, it radioactive, it's this characteristic, scientists can study the progress of certain biological or chemical processes. For example, allow patients to drink a little water containing a very small amount of overweight tea, half an hour later, you can check out the radioactivity from the urine, until 14 days later, radioactive disappear, indicating that the water in the human body residence time is 14 days. If you want to study a chemical process in the context of water, but not allowed to join something else to destroy the chemical reaction, then you can add some water in ordinary water overweight, overweight flow to where, where it appears radioactivity. Scientists can easily use the detector to measure its hiding place.
Heavy water atomic energy industry is an important role, it is the best atomic reactor moderator and the heating agent, after using it, you can greatly reduce the constituent atoms of the fuel. Heavy water is an important national defense materials, the polymerization reaction of the hydrogen bomb is to use it to manufacture, heavy hydrogen at very high temperatures will produce nuclei, where a strong explosion, its energy equivalent to several thousand ten thousand tons of high explosives. An ordinary hydrogen bomb could easily blow up a city. If it explode to release all the energy is converted into electrical energy, humans decades will not be exhausted!
Safety
Hydrogen is the lightest gas known gas. Hydrogen at normal temperature and pressure is a colorless, odorless flammable gas. It addition to lack of oxygen caused by suffocation, but also found no toxicity. Hydrogen and air, oxygen, halogens strong affinity. Hydrogen has a wide flammable range in air and oxygen. Hydrogen high flash point, but its ignition energy is very small, it can catch fire easily, at a small static spark can easily ignite. This is the nature of a special significance. When it can be exposed to heat from fire or combustion, and emit almost invisible flame. Hydrogen is a kind of high-energy fuel, when combined with air or other oxidizers fire way to heat or explosion releasing large amounts of energy, the ferocity of the reaction depends on combustion conditions. A mixture of hydrogen and halogen gas can explode in sunlight. Explosive range of hydrogen and nitrous oxide mixture of 5.2% to 80%, with a mixture of nitric oxide explosion in the range of 13.5% to 49%. This explosive hydrogen gas is extremely dangerous horrible characteristics. Hydrogen is very easy to spread and penetration of gas, it is very prone to leaks, and stays in good ceiling height.
Cryogenic hydrogen at room temperature and the density of hydrogen is different when it starts to evaporate from the liquid hydrogen is heavier than air and deposited on the ground, and so on after the warming began to spread. Cold fog of hydrogen gas can form when moist air encounters, and thus it can be seen in the proliferation of signs. But in the fog periphery can still be seen in the formation of explosive mixtures. If the hydrogen gas in the initial flash evaporation on fire, will produce fireballs.
Highly reducing hydrogen at high temperature with a metal oxide, a metal chloride to free the metal, so it is generally not corrosive. In the role of a catalyst such as platinum and the effect of reducing the organic compound aldehyde unsaturated hydrocarbons.
C2H2 + H2- → C2H4
C2H4 + H2- → C2H6
CH3CHO + H2- → CH3CH2OH
CH3COOC2H5 + 2H2- → 2CH3CH20H
CH3COCH3 + H2- → CH3CH-CH3
| OH
But also between the lattice hydrogen immersion metal lattice expansion or deformation, resulting in a metallic material embrittlement. Steel produces the following decarburization reaction at high temperature and erosion by hydrogen.
Fe3C + 2H2- → CH4 + 3Fe
The transition metal can be hydrogen reversibly adsorbing or absorbing generated variable amounts of metal hydride. The amount of hydrogen is absorbed in the Pd, Pt, Ni, Ti, Fe, etc. are more of Pd, it can absorb 800 times its volume of hydrogen.
Hydrogen slightly soluble in water, the absorption coefficient at 20 ℃ a is 0.0182.
5. Toxicity
Hydrogen itself is non-toxic, it is still the prototype discharge after inhalation. It is a choking agent, but examples in practical work because hydrogen gas suffocation deaths rarely, however, fires and explosions caused by the hydrogen gas has continued to occur. So the explosive nature of hydrogen should arouse enough attention.
Usually after hydrogen filling packed in pressure cylinders or liquefied cryogenic containers. So in addition to the high-pressure hydrogen gas leak risk of fire or explosion, there was a cold liquid hydrogen burn hazard |
|
 |
| 点击数:7461 录入时间:2009/1/2 【打印此页】 【关闭】 |
·上个产品:氮、氮气、Nitrogen, Nitrogen,
·下个产品:PSA氢气纯化设备、PSA hydrogen purification equipment |
|
|
|
|